Posted on by christophepochari
Dramatically reducing the cost of alkaline water electrolyzers using high surface area mesh electrodes, commercial off-the-shelf components, and non-Zirfon diaphragm separators.
Christophe Pochari, Christophe Pochari Energietechnik, Bodega Bay California
The image below is a CAD model of a classic commercial alkaline electrolyzer design, large, heavy, and industrial-scale units. The heavy use of steel for the endplates and tie rods increases the cost of the stack considerably. The above architecture is bulky, and expensive, with high exclusivity in its design and engineering


An example of the excessively elaborate plumbing and alkali water feed and recirculation system, Christophe Pochari Energietechnik had simplified and made more compact this circuitous and messy ancillary system using thermoplastics and design parsimony.

Christophe Pochari Energietechnik thermoplastic lightweight modular quasi stack-tank electrolyzer cell. Our design eliminates the need for heavy end plates and tie rods, since each electrode is an autonomous stand-alone module. Each electrode module is comprised of a hydrogen and oxygen section that sandwiches between the diaphragm sheet placed in the center of the two plastic capsules. Each oxygen and hydrogen capsule contains its respective electrodes. The Pochari electrolyzer cell frame/electrode box is made from injection molding, an ultra low-cost technology at volume. This cell design is highly scalable, modular, convenient, and extremely easy to manufacture, assemble and transport. The culmination of this engineering effort is a dramatic reduction in CAPEX, where material cost, rather than intricate manufacturing and laborious assembly, dominates the cost structure. One of the central innovations that makes our design stand out is the use of active polarity reversal. A series of valves are placed on the oxygen and hydrogen outlet to allow the anode to to be charged as a cathode and vice versa every sixty seconds. This effectively halts the build up of an oxide layer on either the nickel or iron catalyst. Because iron is very close to nickel in its catalytic effectivity for the hydrogen evolution reaction, only a very small performance penalty is encountered when switching from nickel to iron.

20 centimeter diameter COTS electrolyzer stack.
A component breakdown for the core stack, excluding ancillary equipment. Estimate includes material costs only, labor can be factored in later and adjusted for local wage rate differences.
Anode: plasma sprayed nickel mesh or sheet: 4.4 kg/m2 @8000 watts/m2 (4000 w/m2 total current density): $13.7/kW
Cathode: Carbon steel sheet 4 kg/m2: $2/kW
Plastic electrode module and partition frame: $5/kW
Hydrogen oxygen separator: 200-micron polyethersulfone sheet $11/m2: $2.75/kW
EPDM gaskets: $1.5/kW
Total with nickel electrodes: $25/kW
Total with carbon steel electrodes: $11/kW
For electrolyzers to cost significantly above $100/kW, either exotic materials would have to be used, extremely low productivity manufacturing, an inordinate amount of material beyond what is absolutely necessary (ancillary systems), or an extremely low current density. A typical lead-acid battery, with a capacity of 12 volts and 100 amp hours retails for about $70 on Alibaba, or about $60/kW. An alkaline electrolyzer should be manufactured at the same cost as a lead-acid battery and no more. Since nickel is worth roughly $20-25 per kilogram under normal market conditions, and the rest of the electrolyzer is made of very cheap steel and plastic, we can basically exclude the rest of the system for sake of simplicity. Since we’re using 4 and a half kilos of nickel for 8 kilowatts of power output, the price per kilowatt for the single most expensive component of the stack is $11/kW. We have designed our electrolyzer stacks to be 12 inches in diameter and four feet long, weighing approximately 200 lbs for 30 kilowatts of power, the stack is easily moved with a dolly and connecting into a bank of as many electrolyzers as necessary with convenient flexible chlorinated polyvinyl chloride plumbing for caustic water and hydrogen/oxygen. For engineering simplicity, the stack operates at 1 bar and 90 celsius and would use about 50 kWh/kg at 250 milliamps/cm2. Christophe Pochari Energietechnik is developing a tank type electrolyzer design as a viable alternative to the classic filter pressure architecture.
The nearly two-century old technology of alkali water decomposition, with high throughput manufacturing and Chinese production, is ripe for dramatic cost reduction. Current alkaline electrolyzer technology is excessively expensive beyond what bare material costs would predict, imputable mainly due to a production regime which makes use of inordinate customization, procurement of specialized subcomponents from niche suppliers, minuscule production volumes, a noncompetitive market with a small number of big players, and high cost labor. A further contributor to the uncompetitive CAPEX of this low-tech and old technology is the fact that the ancillary and plumbing components that comprise the electrolyzer module system make use of metallic piping and tankage, usually stainless steel or even nickel rather than cheap thermoplastics. A further reason is the choice of a very large stack size (both in terms of diameter and length), which makes manufacturing and transportation that much more challenging and costly. The current manufacturing process for very long electrolyzer stacks requires an adjustable scaffolding or a varying height underground basement with a hydraulic stand, so that the filter press stack can be built up as workers stand at floor levels. Some electrolyzer stacks are as long as 20 feet and can weigh multiple tons, requiring cranes or hoists to move around in the factory. The massive multi-ton stacks are then bolted down from the endplates and lifted out of their vertical assembly position and transported by truck to a site that will require a crane for installation as well. These respective handicaps serve to impose surfeit costs for a technology that is otherwise made up of relatively cost low-cost raw materials and crudely fabricated components with low precious/tolerance manufacturing. Christophe Pochari Energietechnik’ researchers have thus compiled a plethora of superior design options and solutions, using the strategy of consecutive elimination, to finally bring to market affordable hydrogen generators fabricated from readily available high-quality components, raw materials, and equipment procured on Alibaba.com ready to be assembled as small kits ready for use with our novel miniature ammonia plant technology. All of the parts are lightweight and can be lifted by a single person and assembled with common household tools. Our electrolyzers do not exceed 50 kW in size, since our ammonia plants feed off wind and photovoltaics which generate spasmodic current, sundry small electrolyzers are paired up forming a homogenous system, allowing them to be consecutively shot off and on depending on prevailing electrical output, rather than the individual stacks having their power output modulated, which reduces their efficiency. Alkaline electrolyzers require a polarization protection current of around 40-100 amps/m2 during non-operation to mitigate corrosion of the cathode, which is otherwise reduced. An alternative to using a polarization current is simply draining the electrolyte out of the stack, but this would add additional hassle. Most commercial alkaline electrolyzers in operation today are able to fluctuate power output by as much as 125% during a 1-second interval, making it possible to integrate them with wind turbines. During very low-load operation, hydrogen is prone to mix with oxygen by diffusing through the separator membrane when the gas residence time is very high. For this reason, it’s best to operate the electrolyzers at their rated loaded capacity, namely to use our strategy of stacking banks of relatively small units that can be readily shut off and on, rather than throttling a single large scale stack.
Compared to a state-of-the-art lithium-ion battery or Chlor Alkali diaphragm cell, an alkaline cell is an extremely simple and elegant system, consisting of only four major components, each of which features minimal custom fabrication. Alkaline cells, or any electrolyzer for that matter, consists of two basic “architectures”. The most common is the so-called “bipolar” electrolyzer, where current flows from positive to negative through each end of the electrolyzer. The electrolyte serves as the conductor, positive current flows from one end plate until reaching the negative at the opposing endplate, this results in each electrode having a positive and negative on each side. Industrial-scale Alkaline electrolyzers are over 150 years old, with most old fashion designs being constructed entirely out of iron or steel, and corrosion being mitigated not through the use of high-end materials, but by frequent electrode replacement or polarity reversal (to cancel corrosion altogether). In 1789, Adriaan Paets van Troostwijk decomposed water using a gold electrode. The first large-scale use of alkaline electrolysis was in Rjukan Norway, where large banks of electrolyzers fed from the cheap hydropower installations. The Rjukan electrolyzers employed the “Pechkranz electrodes”, invented by Rodolph Pechraknz and patented in Switzerland in 1927, constructed from thick sheets of iron, with the anode electroplated with nickel. It is claimed that the current densities of the Rjukan electrolyzers approached 5500 watts/m2. Most alkaline electrolyzers built before the 1980s used chrysotile asbestos diaphragms.
Prior to the development of the bipolar electrolyzer, most of the early late 19th century designs employed designs that made use of liquid-containing cylinders and submerged electrodes, what is called a “tank type” or “trough” electrolyzer. The electrolyte was contained in a cylindrical vessel, and metal electrodes were suspended from the top. The first modern “bi-polar” electrolyzer was devised by a Russian named Dimitri Latschinoff (also spelled Latchinoff) in Petrograd in 1888, his cell had a current density ranging from 0.35 to 1.4 amp/m2 and used 10% caustic soda. After Latchinoff, a design very close to the modern “filter-press” type was developed by O Schmidt in 1889. Because the Schmidt electrolyzer used potassium carbonate over caustic potash, the electrode corroded at only 1 millimeter per year. In 1902, Maschinenfabrik Oerlikon commercialized the Schmidt bi-polar electrolyzer, which forms the basis for all modern water electrolyzers. The Schmitt design, pictured below, used a cell voltage of 2.5 and generated a hydrogen purity of 99%. The Schmidt electrolyzer generated 2750 liters of hydrogen per hour using 16.5 kilowatts, or 67.34 kWh/kg, or an efficiency of 58.5% of lower heating value. Most early filter press electrolyzers used rubber-bound asbestos diaphragms.

From: The Electrolysis of Water, Processes and Applications By Viktor Engelhardt · 1904


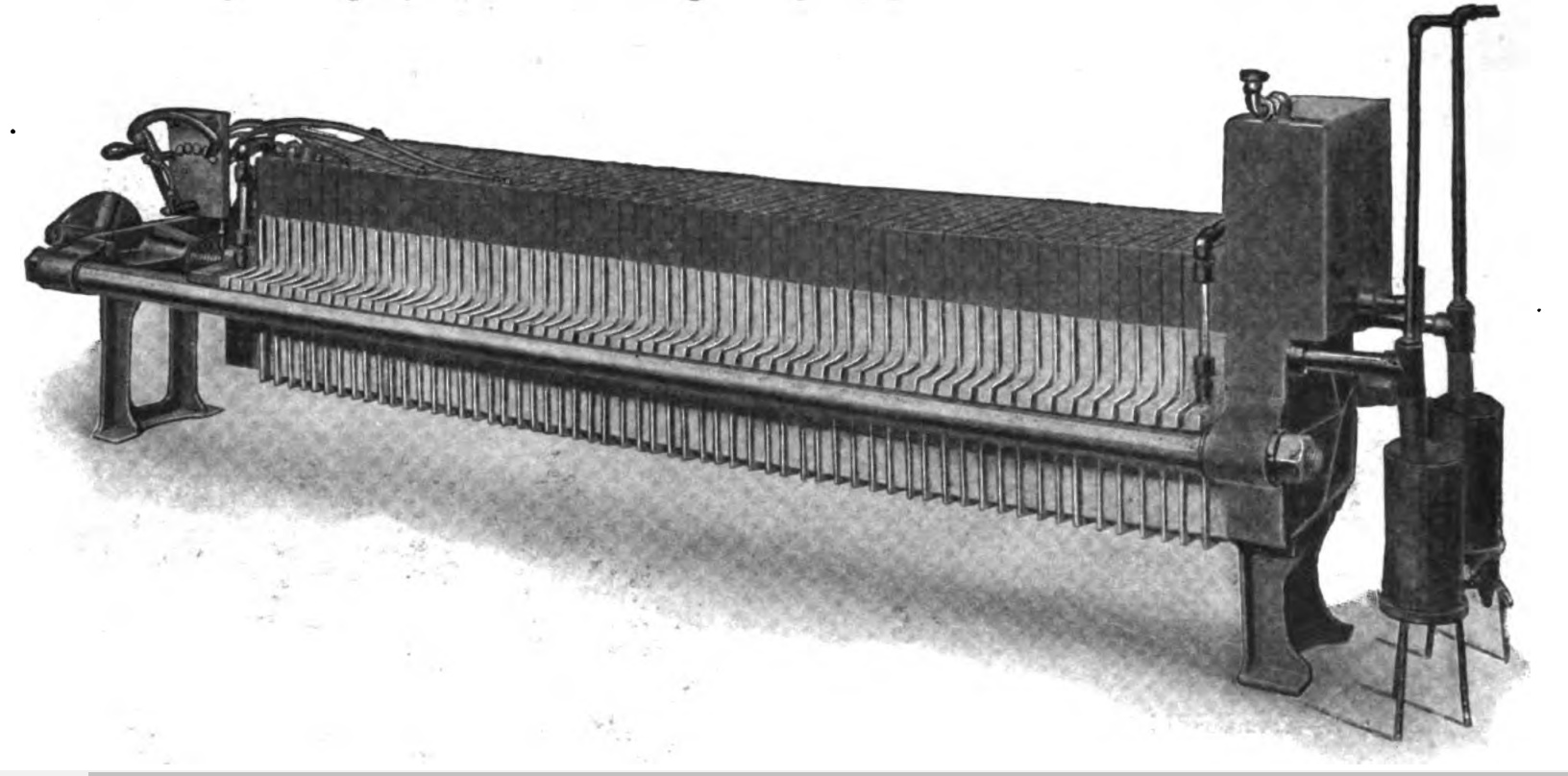
A filter press electrolyzer pictured below was manufactured by National Electrolizer in 1916. The picture above is a filter press electrolyzer made by International Oxygen Company. The picture in the middle is of the asbestos diaphragm pealed in front of the steel electrode. It is claimed in the source that the nickel-plated steel electrodes were “virtually indestructible”. Pictures are taken from the trade journal “Boiler Maker” Volume 16 1916. These electrolyzers were used exclusively for hydrogen welding and cutting of metal. It wasn’t until Rjukan (Norsk Hydro) that this technology first saw use for energetic applications. These Norwegian electrolyzers were also used to electrolyze heavy water for the production of deuterium.

The cost of electrolyzer designs from 1904, notice the Schmidtt filter pressure type cost $182/kW for a 10 kW unit, equal to just under $6000 in 2022 dollars. Prices have declined dramatically since 1904, thanks to more productive labor and manufacturing, more global production of nickel and steel, and more efficient fabrication and machining.
The monopolar electrolyzer energizes each electrode individually with a “rack” or bus bar. This design is rarely used. Bipolar systems are also called “filter press” electrolyzers while monopolar are called “tank type” electrolyzers.
While neither of these designs differs by a significant margin in their performance, the bipolar architecture is considered the most “proven” design and forms the basis of all modern electrolysis technology. The only real disadvantage of the monopolar design is the need for very high current bus bars, since a bipolar will use a voltage equal to the sum of each electrode times the number installed, the current required is greatly reduced, placing less demand on the electrical power supply. For example, if a cell voltage of 2 is used, a hundred electrode pairs allow a high voltage of 100 to be used, while monopolar systems require two volts at each electrode at whatever current is required to provide the power, this would increase electrical losses and generate more heat. The bipolar design is the architecture used in this analysis.

In order to separate the hydrogen from oxygen, a separation or partition plate is used on each electrode, channeling the separated gases into their respective vent holes. The Pochari design differs insofar as the circumferential frame is replaced with plastic, and the design is square rather than round, to make more efficient use of space.

Tank type electrolyzer module
The electrolyzer system is comprised of the stack and the ancillary equipment, which consists of caustic solution storage tanks, pumps, and the hydrogen and oxygen plumbing system. In current alkaline systems marketed by the established players, elaborate plumbing systems are constructed from nickel alloys. To save cost, rather than constructing these components out of stainless steel, they can be made out of high-temperature plastics, which show excellent resistance to caustic solutions. Christophe Pochari Energietechnik is studying how thermoplastics which can withstand moderate temperatures can be used instead to dramatically lower CAPEX. Semi-crystalline plastics: PEK, PEEK, PPS (Polyphenylene sulfide), PA (polyamide) 11/12. Amorphous plastics: PAI (polyamide-imide), PPSU (polyphenylsulfone), PSU (polysulfone), PES (polyethersulfone). Most of these thermoplastics have a density of below 2 grams/cm3, and can handle temperatures over 100 Celsius. The price of Polyphenylsulfone (130 MPa compressive strength), able to operate as high as 150 Celsius, has a density of only 1.3 grams/cm3, with its retail price of $20/kg, it is nearly 7 times cheaper than nickel with equal alkalinity tolerance.
The four components are the following:
#1 Electrodes.
The electrode can consist of any metallic conductive surface, it can be a woven wire mesh, a metallic foam, or a smooth sheet. To achieve the highest performance, a surface morphology featuring a denticulate pattern formed by plasma spraying Raney nickel on the metallic substrate enables a reduction in the “overpotential”, or the excess voltage above the stoichiometric number. In the absence of such a surface finish, a bare metallic surface achieves only minuscule current density.
#2 Gaskets and separators
The gaskets form the seal between the electrode modules preventing gas and liquid from escaping through the edges. The force of the endplates provides the pressure needed to achieve a strong seal. A gasket (made of cheap synthetic rubbers, EPDM etc) is commonly used. EPDM rubber is extremely cheap, around $2/kg. The diaphragm separator is used to prevent the mixing of hydrogen and oxygen to avoid potentially catastrophic explosions from occurring if the rations are within the flammability range of hydrogen, which is 4 to 74% in O2. The diaphragm separator is often the single most expensive component after the electrode. The material for fabricating the diaphragm membrane must be resistant to alkaline solutions, able to withstand up to 100°C, and be selective enough to separate oxygen and hydrogen, while also permitting sufficient ionic conductivity. A number of materials are used, these include composites of potassium titanate, (K2TiO3 fibers, polytetrafluoroethylene (PTFE, as felt or woven, polyphenylene sulfide coated with zirconium oxide, abbreviated Zirfon, perfluorosulphonic acid, arylene ether, and finally, a polysulfone and asbestos composite coating. Commercial electrolyzers make use of an expensive proprietary brand name separator sold by the Belgian company Agfa Gevaert, N.V. The name of this high-end separator is called Zirfon Pearl, and it sells for a huge price premium over the cost of bare polyethersulfone, which itself is a relatively inexpensive plastic that costs around $20/kg in bulk. Many polymers are suitable for constructing separators, such as Teflon® and polypropylene. A commercially available polyethersulfone ultrafiltration membrane, marketed by Pall Corporation as Supor200, with a pore size of 0.2 um and a thickness of 140 microns, was employed as the separator between the electrodes in an experimental alkaline electrolyzer. Nylon monofilament mesh with a size of over 600 mesh/inch or a pore size of 5 microns can also be used. Polyethersulfone is ideal due to its small size, retaining high H2/O2 selectivity at elevated pressures. It can handle temperatures up to 130 C. If polyethersulfone is not satisfactory (excessive degradation rate if the temperature is above 50 C), Zirfon-clones are available to purchase on B2B marketplaces https://b2b.baidu.com for $30/m2 from Shenzhen Maibri Technology Co., Ltd.
#4 Structural endplates:
The fourth component are the “end plates” which consist of heavy-duty metallic or composite flat sheets which house a series of rods tightly pressing the stacks to maintain sufficient pressure within the stack sandwich. For higher pressure systems, such as up to 30 bar, the endplates encounter significant force. In our incessant effort at CAPEX reduction, we have concluded it is possible to cast the endplates rather than machining them, this can reduce their manufacturing cost by 70% relative to CNC machining since investment casting is so much more productive. While we do not plan on focusing on a filter press design, we are still considering developing one as an alternative. Christophe Pochari Energietechnik is also looking into using fiberglass to construct the end plates, at a cost of only $1.5/kg and with tremendous compressive strength, fiberglass is a suitable material, especially for lower pressure stacks operating with no overpressure, therefore placing little to no pressure on the end plates.
Unlike PEM technology, noble mineral intensity in alkaline technology is relatively small, if nickel is to be considered a “noble” metal, then alkaline technology is intermediate. Nickel is not abundant but not rare either, it’s approximately the 23rd most abundant element occurring at a 0.0084% of the crust.
Unlike PEM technology, noble mineral intensity in alkaline technology is relatively small, if nickel is to be considered a “noble” metal, then alkaline technology is intermediate to PEM, but it is difficult to place platinum (50,000-ton reserve) and nickel (100 million ton reserve) in the same category. Nickel is not an abundant element but it is not rare either, it is approximately the 23rd most abundant element occurring at 0.0084% of the crust by mass. If electro-mobility is to gain any degree of traction (which has yet to be proven), deep-sea mining to exploit poly-metallic nodules can be undertaken, doubling the current terrestrial reserves of nickel. It is unfortunate that the nascent modular electrolyzer and miniature ammonia industry, which has yet to amount to anything more than a concept, is forced to compete with wasteful lithium-battery manufacturing for the precious nickel element. We can power cars with cheap steel propane tanks filled with anhydrous ammonia, rather than squandering trillions on elaborate “battery packs” using up precious nickel for the cathodes. Since we are incorrigibly resourceful, we will turn to carbon steel electrodes if market conditions force us to. Nickel prices have been surprisingly stable over time, despite large increases in demand from the stainless steel sector. The market price of nickel has risen only 1.38% a year since 1991. The price of one ton of nickel was $7100 in 1991, equivalent to $14,700 in 2022 dollars, in January 2022, the spot price reached $22,000/ton. At the time of this writing (June 2021), Russia had not yet invaded Ukraine! so while I could anticipate a potential spike in nickel prices, I could not time it, otherwise, everyone would become a billionaire by speculating on the commodity market, and as far as I know, most people have not had much success at that game. In spite of the unfortunate development in the nickel market, the electrode cost is still relatively low even at $50,000/ton, it’s unlikely the Ukraine invasion would cause nickel to rise this much, but it’s possible. It will be important to extensively research carbon steel electrodes if nickel reaches an excessively high price, or increase current density at the expense of efficiency, which we may be able to do thanks to hydrostatic wind turbine technology.
For an alkaline electrolyzer using a high surface area electrode, a nickel mesh electrode loading of under 500 grams/m2 of active electrode surface area is needed to achieve an anode life of 5 or more years assuming a corrosion rate of below 0.25 MPY. With current densities of 500 milliamps/cm2 at 1.7-2 volts being achievable at 25-30% KOH concentration, power densities of nearly 10 kW/m2 are realizable. This means a one-megawatt electrolyzer at an efficiency of 75% (45 kWh/kg-H2 LHV) would use 118 square meters of active electrode surface area. Assuming a surface/density ratio of a standard 80×80 mesh, 400 grams of nickel is used per square meter of the total exposed area of the mesh wires. Thus, a total of 2.25 kg of nickel is needed to produce 1 kg of hydrogen per hour. For a 1 megawatt cell, the nickel would cost only $1000 assuming $20/kg. This number is simply doubled if the TBO of the cell is desired to increase to 10 years, or if the power density of the cell is halved. Christophe Pochari Energietechnik is planning on using carbon-steel electrodes or plain iron electrodes to replace nickel in the future to further redux CAPEX below $30/kW, our long-term goal is $15/kW, compared to $500 for today’s legacy system from Western manufacturers. Carbon steel exhibited a corrosion rate of 0.66 MPY, while this is significantly above nickel, the cost of iron is $200 per ton (carbon steel is $700/ton), while nickel is $18,000, so despite a corrosion rate of at least 3x higher, the cost is 25x lower, yielding of 8.5x lower for carbon steel. The disadvantage of carbon steel despite the lower CAPEX is decreased MTBO (mean time before overhaul). Christophe Pochari Energietechnik has designed the cell to be easier to disassemble to replace the corroded electrodes, we are also actively studying low-corrosion ionic liquids to replace potassium hydroxide. We are actively testing a 65Mn (0.65% C) carbon steel electrode under 20% KOH at up to 50 C and experiencing low corrosion rates confirming previous studies. Christophe Pochari Energietechnik is testing these carbon steel electrodes for 8000 hours to ascertain an exact mass loss estimate.
What kind of current density can be achieved by smooth plates?
Current densities of 200mA/cm2 at 1.7 volts (3.4 kW/m2) generates an efficiency of 91% even with non-activated nickel electrodes.
If a corrosion rate of 0.10 MPY is chosen, which is very conservative, then for a material loss rate of 5% per year, 400 grams per square meter is required, yielding a cost per kW of $4.7. If one desires to be extremely conservative, imagine an electrode is used that is around 1 millimeter thick. Since only the anode requires nickel (the cathode can be made of steel since it’s being reduced), we will use 3.9 kg of nickel sheet for 1 square meter, since the current density is 3600 watts per/m2 (200 milliamps x 1.8 volts), and this number is doubled since only half the electrode is nickel, the price per kW is $21. This illustrates that even if the designer wants to use an extremely thick electrode, far thicker than necessary, the cost of the number one most materially sensitive component is only 2 percent of the cost of present commercially available electrolyzers, suggesting chronic manufacturing and production inefficiency among current producers.
Corrosion is by far the single biggest enemy of the electrolyzer, it’s an issue that’s under-discussed but accounts for the preponderance of performance degradation. All metals, even noble ones, tend to oxidize over time. The anode, the negative side, the electrode that evolves hydrogen and is constantly being oxidized, and turns black within hours of use. The oxygen generating is subject to reduction and remains shiny no matter how long it is exposed to the alkaline environment. The hydrogen electrode experiences immense oxidative pressure, and will rapidly accumulate a black oxide layer, in the case of nickel, the oxide layer is comprised of nickel hydroxide. No material is lost, and it’s theoretically possible to recover all of the metallic nickel from the oxide layer which eventually is lost in the alkaline medium. On the oxygen electrode, the black oxide layer quickly reaches a peak and begins to pacify it and slow down the rate of further oxidation, but at the expense of electrochemical performance.


For a lower corrosion rate of 1 um/yr, a total mass loss of 7% per year will occur with a surface/mass ratio of 140 grams/m2-exposed area, the nickel requirement is only $350 or 17.5 kg for one megawatt! Although this number is achievable, higher corrosion rates will likely be encountered. To ensure sufficient electrode reserve, a nickel loading of around 400-500 grams/m2 is chosen. Pure nickel experiences an excessively high corrosion rate when it is “active”, it becomes “passive” when a sufficient concentration of iron (NiFe2O4), or silicate is found in the oxide layer. For Incoloy alloy 800 with 30% Ni, 20% Cr and 50% Fe experiences a corrosion rate of 1 um/yr at 120 C in 38% KOH, pure nickel is over 200 um. “The “active” corrosion of nickel corresponds to the intrinsic behavior of this metal in oxygenated caustic solutions; the oxide layer is predominantly constituted of NiO at 180°C and of Ni(OH) 2 at 120°C. The nickel corrosion is inhibited when the oxide layer contains a sufficient amount of iron or silicon is present”. The results drawn from this study indicates the ideal alloy contains around 34% Ni, 21% Cr, and 45% Fe. The cost breakdown for the three elements are $18/kg, $9/kg and $0.2/kg, giving an average of $8.1/kg. For a passive corrosion rate of 1 um/yr, a 10% annual material loss corresponds to an electrode mesh loading of 90-100 grams/m2, or $0.11/kW. That is 11 cents per kW! This does not include mesh weaving costs. A 600 mesh weaving machine costs $13,000. The conclusion is meshing costs are very minimal, less than a few cents per square meter.
For the diaphragm separators using a 200 um thick sheet of polyethersulfone (PES), around 20 grams is used per kilowatt, at a typical cost of PES of $25/kg assuming density of 1.37 g/cm2, the cost would be around $0.50/kilowatt assuming an electrode power density of 6.8 kW/m2 (400 milliamps at 1.7 volts). Since Christophe Pochari Energietechnik always adheres to COTS methodology, the expensive and specialized Zirfon membrane is dispensed with in favor of a more ubiquitous material, this saves considerable cost and eases manufacturability as the need to purchase a specialized hard to access material is eliminated. Gasket costs are virtually negligible, with only 4.8 grams of rubber needed per kilowatt, EPDM rubber prices are typically in the range of $2-4/kg. For 30% NaOH at 117 C, a corrosion rate of 0.0063 millimeters per year (0.248 MPY) is observed for an optimal nickel concentration of 80%. This means 55 grams of Ni is lost for one square meter, if we choose 10% per year as an acceptable weight loss, we return to 550 grams per square meter as the most realistic target nickel loading, with much lower loading achievable with reduced corrosion rates. A lower concentration of KOH/NaOH and lower operating temperature can be utilized as a trade-off between corrosion and power density. The total selling price of these units cost including labor and installation is $30/kW. In 2006, GE estimated alkaline electrolyzers could be produced for $100/kW, clearly, must lower prices are possible today. At an efficiency of 6.5 MMW (47.5 kWh/kg-H2), the price is $1430/kg-hour. After the cell stack costs, which we demonstrated can be made very minimal with the COTS design philosophy, the second major cost contributor is the power supply. For a DC 12 volt power supply, $50 is a typical price of a 1000 watt DC power module. Thus, to summarize, alkaline electrolyzer material costs are effectively minuscule, and the cost structure is dominated by conventional fabrication, assembly, and electrode deposition techniques as well as the power supplies and unique requirements of low voltage direct current high amperage power. High-efficiency DC power supplies cost as little as $30/kW and last over 100,000 hours. Once components can be mass-produced and assembled with as little use of manual labor, costs can be brought down close to the basic material contribution. The only uncertainty for the future of alkaline electrolysis is the price of nickel, certain disruptions in the supply of nickel could make the technology less competitive, as long as carbon steel electrodes are unproven. When this text was written, the author has purchased $2000 worth of nickel sheets on Alibaba when the spot price was $18/kg.
It should be noted the activity of the nickel electrode depends heavily on its morphology. A smooth sheet has very little activity and is thus not suitable for industrial scales, although, for small electrolyzers, a smooth catalyst can be sufficient if power density is not an exigency. Catalysts activity depends not on the total surface area available exposed to the reactant material, rather, catalyst activity depends almost exclusively on the presence of so-called “active sites” or “absorption sites” comprised of kink sites, ledges, and steps, adatoms, and holes. These sites, characterized by local geometric perturbation, account for effectively all the activity of a catalyst. It can be said that the vast majority of the catalyst area is not active. By achieving a high fraction of active sites, the current density holding voltage constant can be increased 10-fold. Raney nickel catalysts were first invented in 1948 by Eduard W. Justi and August Winsel. A properly leached Raney nickel catalyst can attain an immense surface density of 100 m2/g.
Raney nickel, an alloy comprised of aluminum and nickel, is sprayed on the bare nickel sheets, meshes, or nickel foam, forming an extremely high specific surface area by producing micron-size jagged edge clumps. This process is called sputtering deposition. The high velocity and temperature of the metal particle cause them to mechanically adhere to the nickel surface. During the application of the Raney nickel with the plasma spraying machine, it is important for the distance, temperature, and deposition rate to be fine-tuned, to avoid excessively thick deposition or clumping. Examination with electron microscopes can be performed by sending a sample of the piece to an electron microscope rental service. After the material has cooled and solidified, the aluminum is then leached and extracted from the surface using a caustic solution, leaving the pure nickel electrode ready to be used. This leaching process, where the aluminum is pulled away from the nickel surface, is what leaves the spongy-like surface and contributes to the stellar electrochemical activity of Raney nickel electrodes. Raney nickel sells for around 300 RMB per kg, or about $50/kg on https://b2b.baidu.com. By mass, only a tiny fraction of the electrode is comprised of the Raney nickel, a thin heterogeneous layer, usually far less than 100 microns. The primary cause of electrode degradation is the loss of the high surface area active sites through the absorption of nickel oxide on the outer surface. Corrosion is almost impossible to prevent, but since no material is lost, the electrodes can simply be regenerated after their useful life. A simple yet elegant option to slow down or even arrest altogether electrode degradation is by periodically reversing the polarity. In doing so, the soon to be oxidized anode has its nickel oxide stripped off by turning it into a cathode and transferred to the former cathode, this allows each electrode to remain at a relatively new state, any accumulated nickel oxide on the hydrogen side is removed after 24 hours. The power supply can simply feature a polarity reversing switch, by installing a mechanical buss-bar which manually moves the input current from positive to negative, requiring no modification to the standard switching power supply. The only tedious aspect of this design is the need to mechanically switch the hydrogen and oxygen hoses, but this too can be done with automatic valves which simply re-route hydrogen into the former oxygen hose and vice versa. Oxy-hydrogen cutting torch operators employ this method to increase the life of their stacks. By employing a simple yet novel solution to corrosion prevention, plain steel anodes can be reliably used. Youtuber NOBOX7 reverses the polarity on his homemade HHO cutting torch generator.
“The reduction in corrosion due to periodically reversed currents appears to be due to the fact that the corrosive process is in a large degree reversible; so that the metal corroded during the half-cycle when current is being discharged is in large measure redeposited during the succeeding half cycle when the current flows toward the metal. This redeposited metal may not be of much value mechanically, but it serves as an anode surface during the next succeeding half cycle, and thus protects the uncorroded metal beneath. Effect of frequency on rate of corrosion: The corrosion of both iron and lead electrodes decreases with increasing frequency of reversal of the current. The corrosion is practically negligible for both metals when the period of the cycle is not greater than about five minutes. With iron electrodes a limiting frequency is reached between 15 and 60 cycles per second, beyond which no appreciable corrosion occurs. No such limit was reached in the lead tests, although it may exist at a higher frequency than 60 cycles. The corrosion of lead reaches practically the maximum value with a frequency of reversal lying between one day and one week. The corrosion of iron does not reach a maximum value until the period of the cycle is considerably in excess of two weeks”.
Digest of Publications of Bureau of Standards on Electrolysis of Underground Structures Caused by the Disintegrating Action of Stray Electric Currents from Electric Railways, United States. National Bureau of Standards, Samuel S. Wyer · 1918
“According to experiments by Larsen, daily reversals of polarity reduce the electrolytic action to one fourth, and hourly reversals to one thirtieth of its normal value . The changing of the direction of the current causes a partial restoration of the metal which has been removed, this effect increasing with the frequency of the reversals. Also, according to Larsen, the nature of the electrolytic action is less harmful when the polarity is periodically reversed than when it remains always the same. When the current flows continuously in the same direction, the pipes become deeply pitted, but when the polarity is periodically reversed the corrosion is more widely and uniformly distributed. Therefore, in all cases where the conditions permit, it is advisable to reverse the polarity of the system at certain intervals. The hourly reversal of polarity reduces corrosion to a very great extent, but when alternating current, even of low frequency, is used the corrosion is completely done away with”.
Stray Currents from Electric Railways by Carl Michalke · 1906
The most challenging aspect of manufacturing a high-performance alkaline electrolyzer is catalyst preparation. Manufacturing an electrolyzer is not semiconductor photolithography, it is a delicate process, but by no means a proprietary or high-tech procedure. The equipment required to perform electrode manufacturing is not specialized, but dual-use, with commercial systems being readily available for electrolyzer manufacturing, obviating the need for expensive and niche suppliers. The major electrolyzer manufacturers do not possess any special expertise that we cannot acquire ourselves. Plasma spraying is the most common method to achieve a highly denticulate surface. A plasma spraying torch can be procured for around $2000 and used to gradually coat the smooth nickel sheets with a highly porous and ragged surface with the Raney nickel. The HX-300 thermal spraying machine sold by Zhengzhou Honest Machinery Co Ltd, runs at 300 amps DC, has a duty factor of 60%, and costs only $1850. It can spray a multitude of metal powders at 0.6 megapascals of pressure.

A typical thermal spraying machine, used to apply heat-resistant coating for automobile components and many disparate applications. These machines require a flow of coolant and compressed air to operate. Their average price is between $2000 and $10,000.
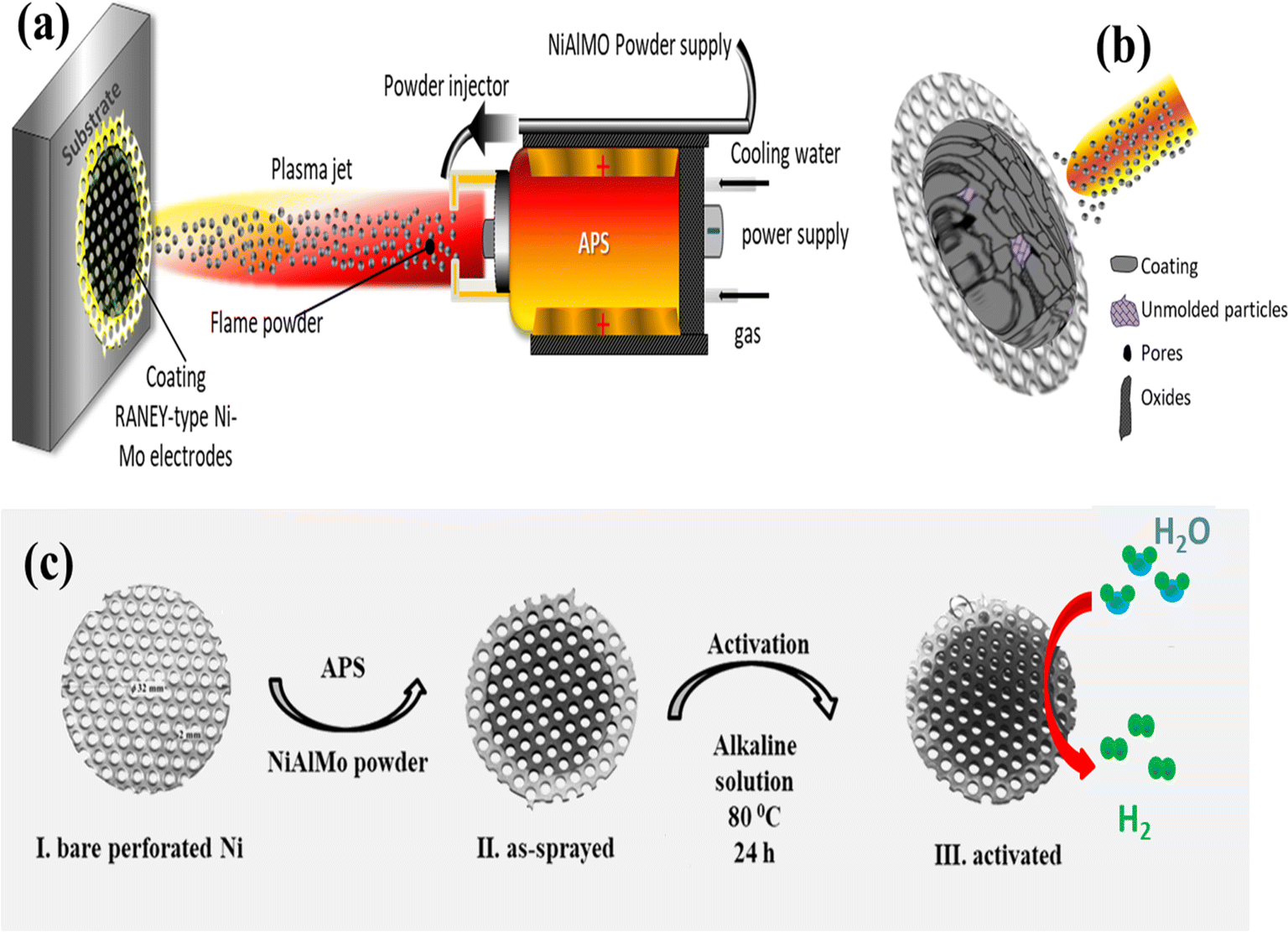
Bare sheets of smooth nickel would be placed on the floor and either a manual operator or gantry frame can be used to automatically pass the plasma head across the metal surface, in the same manner that a painter applies paint over a surface. This process is called sputtering deposition. After the material has cooled and solidified, the aluminum is then leached (extracted) from the surface using a caustic solution. In the paper “Plasma spraying can be done either in a vacuum or in an atmospheric environment. Electrochemical characterization of Raney nickel electrodes prepared by atmospheric plasma spraying for alkaline water electrolysis, the authors Ji-Eun Kim et al achieved satisfactory results using a standard atmospheric plasma thermal spraying machine using Raney nickel particles 12 to 45 microns. Christophe Pochari Energietechnik is developing a low-cost plasma spraying machine using ubiquitous microwave components to perform catalyst preparation, but such an option is only of interest to hobbyists and the HHO energy community, since any commercial-grade factory would be able to purchase a standard thermal spraying machine. Once catalyst surface preparation is complete, the electrolyzer is ready to assemble. Commercial plasma deposition where Raney nickel microparticles are blasted onto a smooth nickel mesh and high temperature and high velocity have an inherent drawback: they produce a brittle adherence, the adhesion between the leached Raney nickel microparticles and the underlying smooth substrate is poor and prone to cracking and peeling.
.
The polyethersulfone diaphragm separator and rubber gaskets can be cut precisely into circular pieces with a laser cutter, along with the nickel sheets, using virtually no labor other than what is required to load the sheets onto the laser cutting machine bed. Then, once all the parts have been cut, prepared, and readied for installation, the low-skill process of stacking these components and the bolting of the endplates, plumbing fittings, etc can be performed in low labor cost countries, such as Mexico. The electrolyzer can also be packaged as easy to assemble kits, so that owners can perform assembly themselves, further saving cost.



Achievable current densities for a number of alkaline electrolyzers.

180 C at 38% wt KOH at 4 MPa Oxygen

150 C at 38% wt KOH at 4 MPa Oxygen

120 C at 38% wt KOH at 4 MPa Oxygen


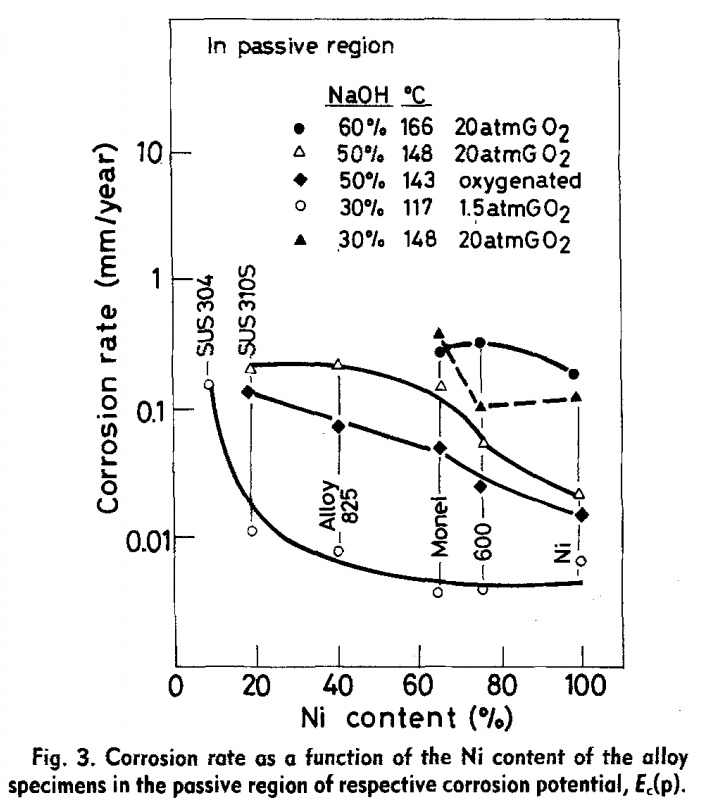





Typical alkaline electrolyzer degradation rate. The degradation rate varies from as little as 0.25% per year to nearly 3%. This number is almost directly a function of the electrocatalyst deactivation due to corrosion.

Diaphragm membrane rated for up to 100 C in 70% KOH for $124/m2: $8.8/kW

*Note: Sandvik materials has published data on corrosion rates of various alloys under aerated sodium hydroxide solutions (the exact conditions found in water electrolyzers), and found that carbon steel with up to 30% sodium hydroxide provided temperatures are kept below 80 Celsius.

Great to see you back focussed and all the progress you have made in H2/Ammonia/Etc It will take me some time to read through it all. It mostly looks like design, any prototypes? John
LikeLike
Hi John. Recently, I’ve added someone on board to help with technology development. We’ve recently made tremendous progress on discovering new alloys that can enable ruthenium-free catalysts. We’re very close to a prototype for ammonia decomposition. Is there a platform we can chat, Skype or Zoom?
LikeLike
I was very informative…regarding cost reduction of Alkaline electrolysers..i intend to discuss further in detail..any possibility to connect you through virtual mode
LikeLike
Hi Rahul, let’s talk on skype
LikeLike
Very interested in learning more.
LikeLike
Hi Christophe, What is the status now, is it commercialized?
LikeLike
Hi Amol, all of the technologies featured on my website are not yet commercialized, hence substantiating the case made for their novelty.
LikeLike
Hello Christophe, I am developing an electrolytic cell. I am right now designing the stack dimensions and I am considering using nikel foam together with stainless steel electrodes. Would like to know if it is possible to arrange a meeting to talk about the system I am designing?
Thanks you.
LikeLike
please send me an email
LikeLike
we are interested to partner with you for the technology enabling economic production of Green Ammonia
Please contact us for collaboration.
Let me have your email + telephone contact.
LikeLike